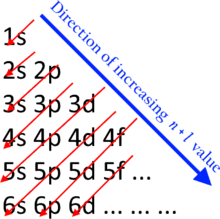
Periodic table - Noble Gases, Pauli Exclusion Principle, Aufbau Principle, Shell System | Britannica
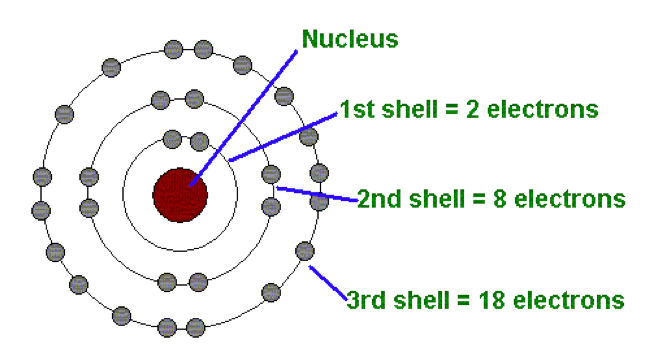
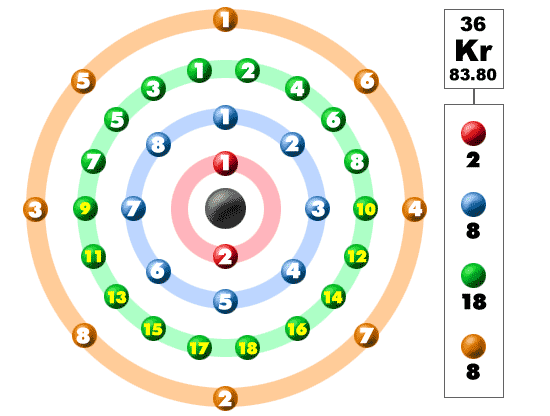
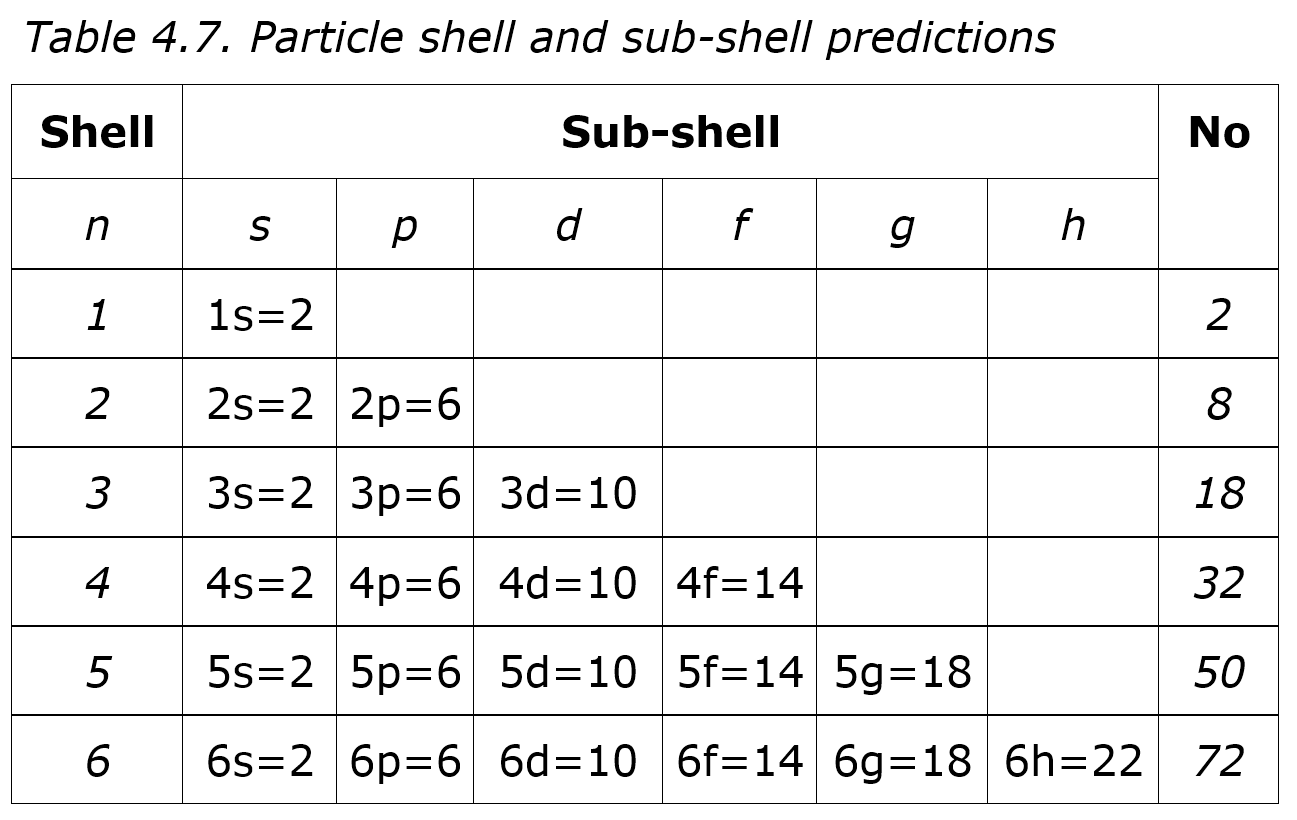
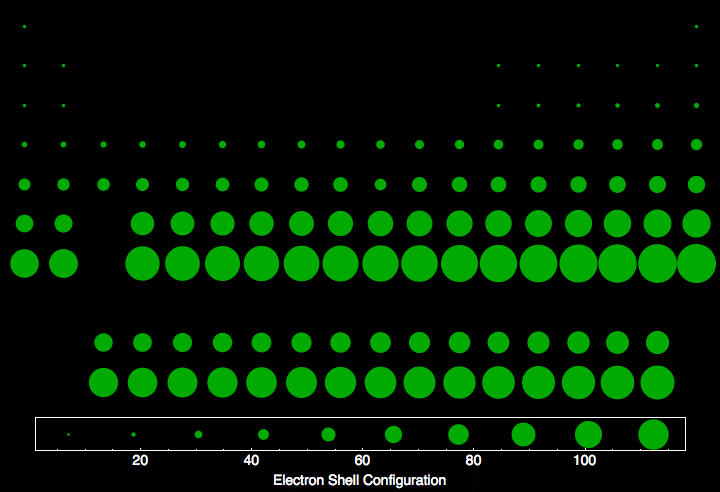
Why are the electrons of potassium 2 8 8 1, instead of 2 8 9, since the M- shell has a maximum of 18 electrons (according to 2n^2)? - Quora
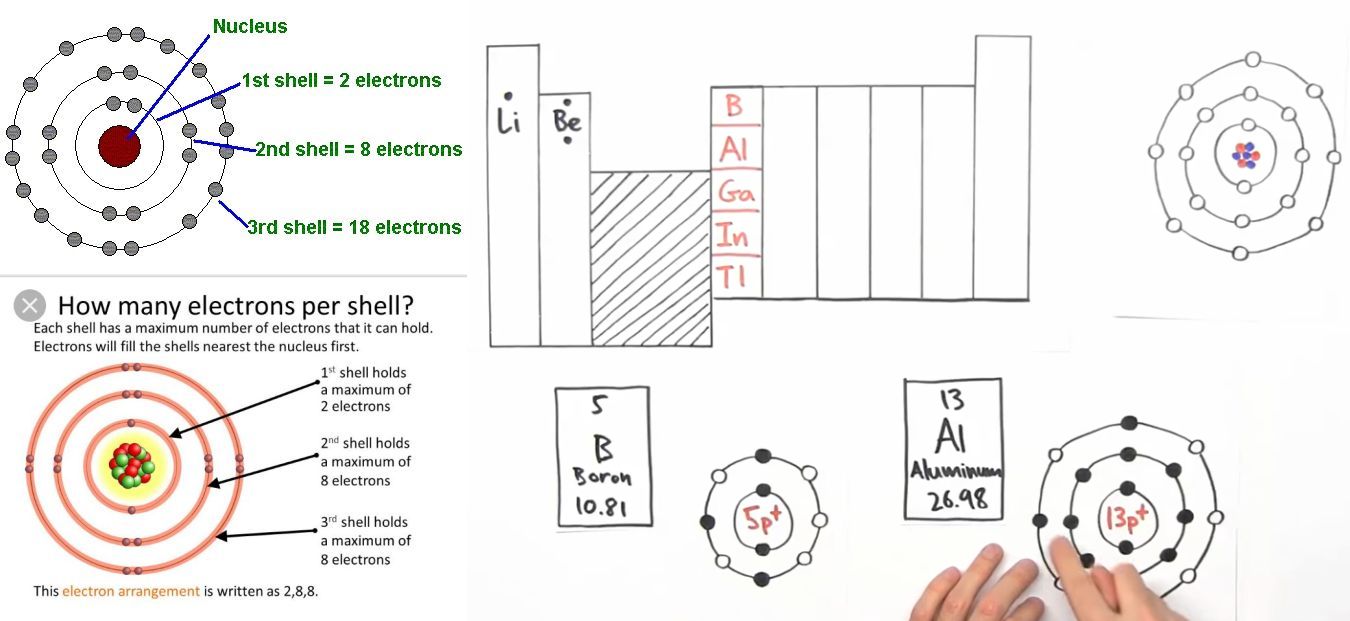
I'm confused. Common knowledge (and almost all images on the internet) says the electron shells/levels hold 2, 8, 18, 32, etc. in ascending order. Now I am being instructed the shells hold

N shell can hold upto 32 electrons so why are there sometimes only 18 in it and the rest are - Chemistry - - 11751065 | Meritnation.com